Establishment and Application of ISO International Standard System for Chinese Medicinal Materials
LIU Liang, ZHOU Hua, HUANG Yu Feng, XIE Ying, HE Fan
Macau University of Science and Technology
At the background of economic globalization and technological integration, standards have become the commanding heights of economic and technological competition. The International Organization for Standardization (ISO) is the world’s largest and most authoritative voluntary international standards research and development organization. Its development and certification standards play a vital role in enhancing the product quality and safety, breaking down the technical barriers, and promoting the international trade. The development of international standardization of traditional Chinese medicine and the promotion of international exchanges and dissemination of traditional Chinese medicine have become one of our national strategies. This project relies on the platform advantage of the Macau University of Science and Technology undertaking the secretariat of "Working Group on Quality and Safety of Raw Medicinal Materials and Traditional Processing" in the International Organization for Standardization of Technical Committee of Traditional Chinese Medicine (ISO/TC249/WG1) to formulate, develop, coordinate and publish a series of ISO international standards for Chinese medicine. The project established the ISO international standard system for Chinese medicine, realized a major breakthrough in the international standard of Chinese medicine, guided the international standard of Chinese medicine to the fast track of sound development, and launched the new label of "Made in Macau" for the ISO international standard of Chinese medicine. At the same time, it is also an important starting point for implementing the national strategy of "Development Planning Outline of Guangdong-Hong Kong-Macao Greater Bay Area", creating a high-level high-tech carrier and platform for Chinese medicine to go global in the Hengqin Guangdong-Macao cooperative Chinese medicine science and technology industrial park.

Fig 1 The Chairman presented the convenors with a renewal certificate at the 9th ISO/TC249 plenary meeting (the second right Chair professor Liu Liang)
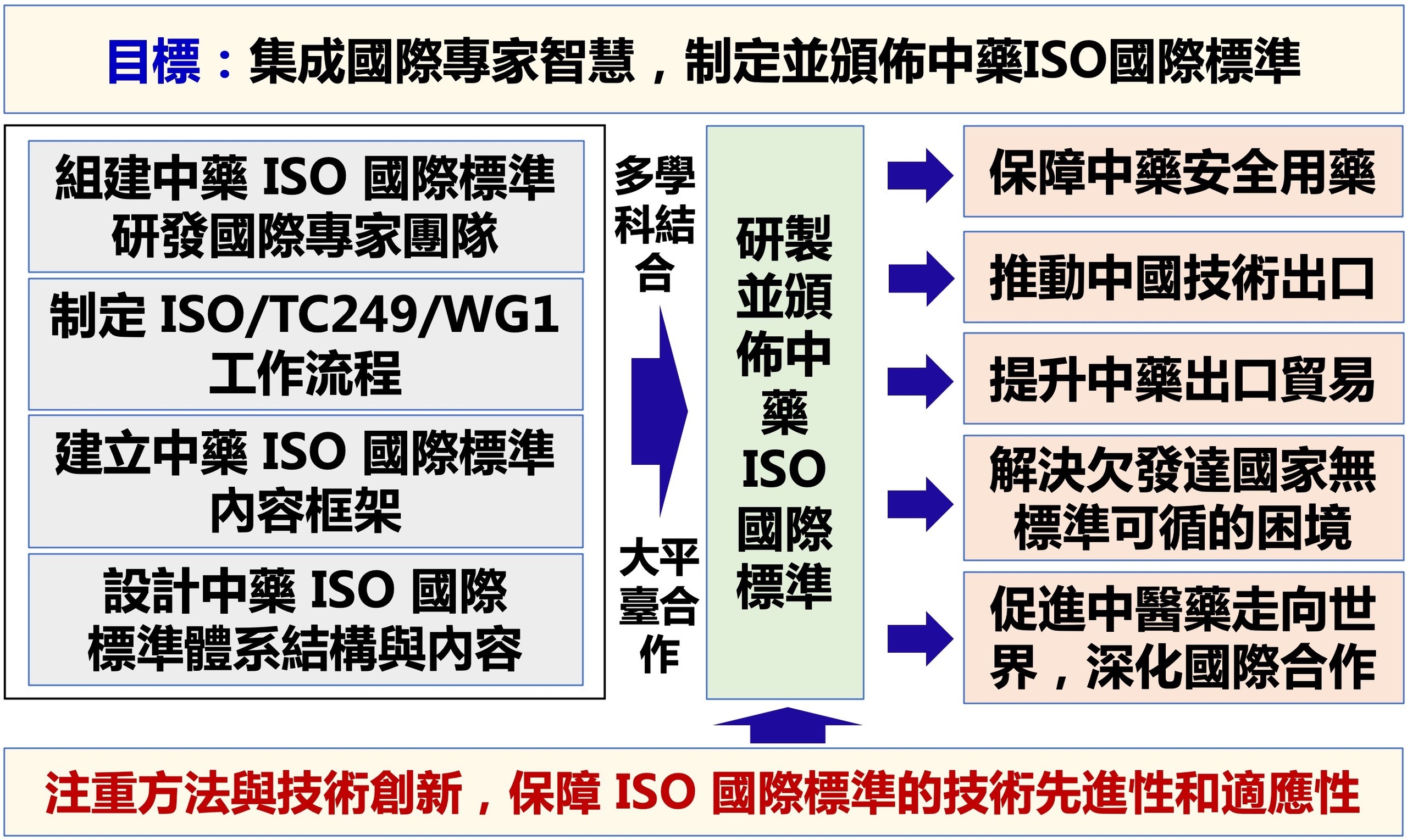
Fig 2 Strategy to develop ISO international standard system for Chinese medicines and promote organized international trade of Chinese medicines
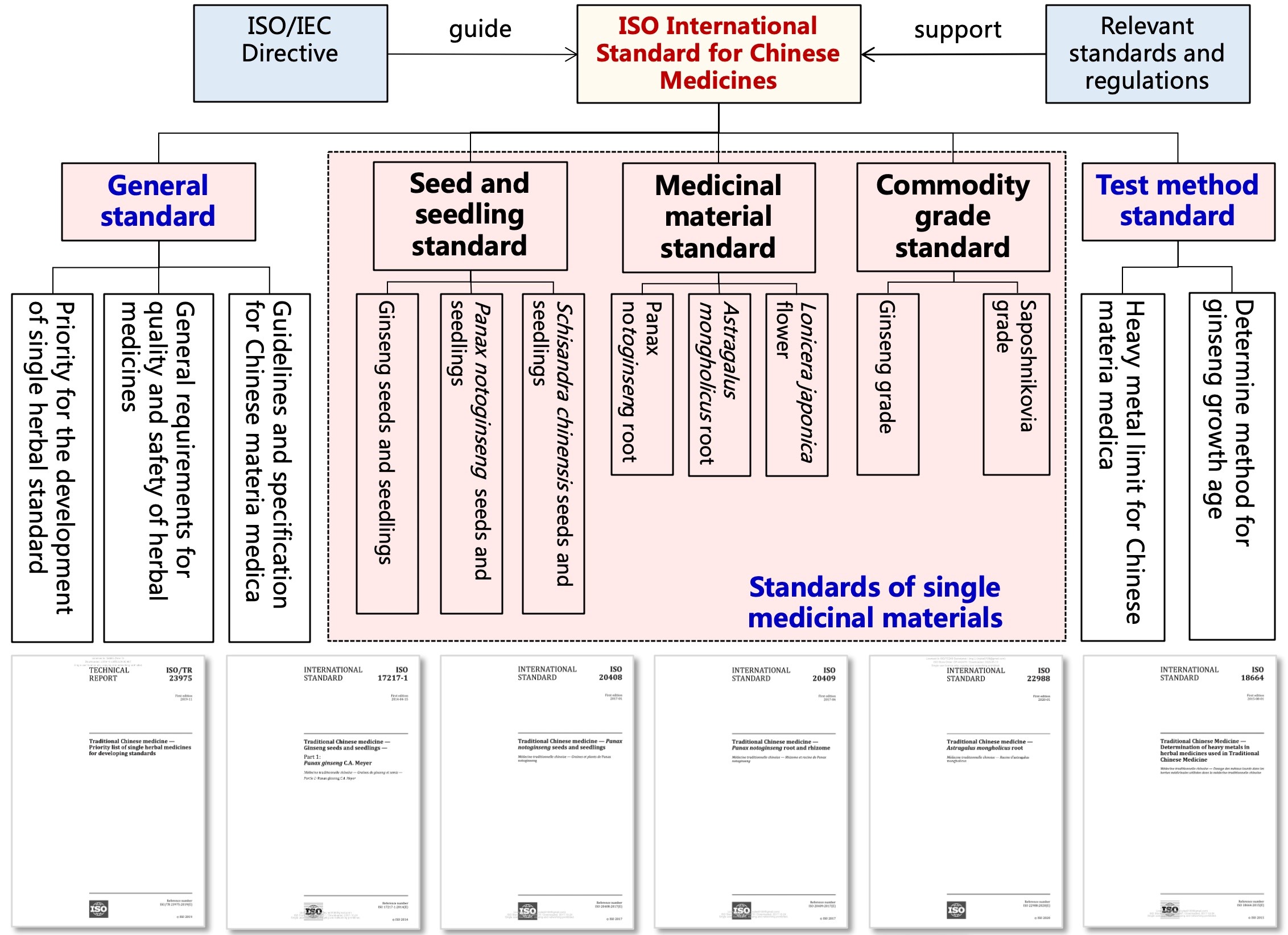
Fig 3 The ISO international standard system for Chinese medicines consisting of three levels, i.e. general standards, single medicinal material standards, and method standards


